|
|
|
Testosterone, oestrogen and progesterone are all steroids derived from cholesterol. They are lipid soluble and diffuse into cells where they can, e.g. attach to proteins or act as precursors for other androgens. Sex hormones can act on neurotransmitter function directly, e.g. altering synthesis, release or reuptake, or indirectly attaching to DNA and increasing protein synthesis (could take minutes or hours).
Androgens are converted to oestradiol mainly in the hypothalamus and limbic system. Testosterone enters the target cell and is converted to oestradiol.
They are reduced to other androgens mainly in the pituitary gland and brain stem.
The receptors (sometimes in the cell nucleus) are oestrogen receptors. In both sexes, there are heavy concentrations of oestradiol receptors in the preoptic area and hypothalamus, and the amygdala. Oestrogen receptors have also been found in the cortex. This pattern is the same in males and females. It enters the cell and influences genomic activity.
Masculinisation involves both oestrogen and androgen receptors, as evidenced by blocking the testosterone-oestradiol conversion.
Oestrogen receptors must remain unoccupied.
If females are treated with testosterone prenatally, they are masculinised. DES, the synthetic oestrogen, has similar effects but the nuclei don’t grow quite as large, but nearly as large.
Tamoxifen blocks the action of oestrogen.
Cyproterone acetate blocks the action of testosterone.
Males treated with cyproterone acetate show normal masculinisation, but with tamoxifen they are demasculinised, so oestradiol causes the masculinisation.
Females aren’t masculinised because foetal alpha-foeto-protein in the blood (synthesised in foetal liver) mops up the maternal oestrogens (not testosterone). DES is a synthetic oestrogen that can enter the brain and mothers treated with it had masculinised daughters.
Sexually dimorphic characteristics are those characteristics that differ from males to females. Genes on the short branch of the Y sex chromosome control sexually dimorphic characteristics, so if your sex chromosomes are all Xs you’ll turn out female, and if you’ve got 1 Y or more you’ll turn out male.
Foetuses’ gonads differentiate around the 6th week of gestation because a gene on the short branch of the Y chromosome creates a testes-determining factor enzyme that turns the gonads into testes. That’s the last thing the genes do in terms of sexual differentiation, and thereon it’s under hormonal control.
The critical period for these gonadal steroids is the first trimester (3 months) of pregnancy. It’s perinatal (around birth) in the rat, from 4 days before birth to 10 days after, because rats are born more premature than humans.
Before 6 weeks you have 2 sets of tubes attached to the testes, a Müllerian duct that can turn into the female reproductive system, and a Wolffian duct that can turn into a male reproductive system. The testes produce 2 hormones: Müllerian-duct-inhibiting-substance that cause the Müllerian duct to degenerate, and testosterone that makes the Wolffian duct develop into the male reproductive system. If these hormones aren’t around the gonads turn into ovaries at around 12 weeks because of maternal oestrogen, and the Wolffian duct automatically degenerates and the Müllerian duct grows into the female reproductive system. From 8-12 weeks, if there’s testosterone you grow male external genitalia, if there isn’t you grow female.
Removing gonadal tissue from foetal rabbits, which later developed oviducts, uterus, vagina and cervix, showed this. If they are injected with oestrogen and progesterone as adults, they exhibit lordosis. In contrast, males castrated later in development they don’t develop female genitalia and exhibit lordosis.
Congenital adrenal hyperplasia is a condition where the foetal adrenal gland produces significant amounts of male steroids=androgens. If this occurs say after the 6th week of life when the genitalia are differentiating then female foetal genitalia will become masculinised. Females’ behaviour is also often masculinised by foetal exposure to androgens. A similar effect is found if the mother takes anabolic steroids early in the pregnancy.
The effect was first found in monkeys, where injecting pregnant mothers with testosterone was found to cause female infants to have male genitalia. Prenatally androgenised monkeys also show more rough-and-tumble play than normal. They will be more aggressive and show male mating behaviour as adults as well as lordosis. If injected with testosterone as adults, their mounting behaviour is equivalent to a male’s. Pseudo-hermaphrodite refers to the fact that the Müllerian duct derivatives (no Müllerian inhibiting substance) are present, but the genitalia aren’t.
In humans the androgen levels often become high later too late to masculinise the Wolffian duct, so pseudo-hermaphrodites will have a normal female reproductive system, but fusing of the labia and an enlarged clitoris. Sometimes the sex of the child is misidentified, yet they lead normal lives as boys till adolescence when sexual development and identification problems occur. Humans with adrenogenital syndrome are more tomboyish, more likely to play with trucks rather than dolls, as kids and more likely to be lesbians or bisexual as adults.
Pseudo-hermaphroditism also occurs in males as androgen insensitivity syndrome. They have the testes determining factor gene so develop testes. Though the hormone production of the testes is normal, the target tissue androgen receptors are insensitive to it. Because the testes still produce Müllerian inhibiting substance, the Müllerian duct degenerates and they do not develop a female reproductive system. They won’t have a uterus and oviducts. Because of the lack of sensitivity to androgens, they will develop female genitalia.
They still have oestrogen receptors. At puberty, they develop female secondary characteristics because of oestrogens produced by their adrenal glands and testes. Due to the lack of a female reproductive system, they will not menstruate. But, as some of the testosterone gets converted to oestrogen, they will develop female secondary characteristics like breasts. Behaviour too is feminised by lack of sensitivity to testosterone, and they often lead normal female lives apart from not being able to have kids.
Additional chromosomes increase mitotic cycle duration; absence of one X shortens it.
In Turner’s syndrome the sperm loses its sex chromosome, the foetus is left with a single X chromosome. The ovaries bud at 12 weeks, then atrophy. Wolffian ducts decay. Maternal oestrogen means the Müllerian duct will develop into a female genital tract. Gender identity normal. The lack of ovaries shows up at puberty when you get a failure to develop secondary characteristics: there’s no growth spurt, breast development, menstruation… They have immature appearing normal female genitalia. They have an infantile cervix and uterus. In addition they may have other problems, e.g. heart problems, neck webbing. If they’re treated with ovarian hormones at puberty they will develop normally. Their gender identity is the same as normal females. Treatment includes growth hormone and low doses of oestrogen.
The male version, where the egg loses its sex chromosome does not occur because single Y embryos do not survive.
Klinefelter’s syndrome occurs when a male has an extra X chromosome, frequently derived from the mother. This causes the testes to develop abnormally, hence inadequate levels of pubertal androgen and fewer sperm. This increases their feminine characteristics, though the effects are not usually seen till adulthood. They’ll be tall, maybe with breasts and poor muscular development. They’re often infertile, with low sexual drive or impotence, and they may lack drive/ambition or be mildly retarded.
Normal puberty. Males can be born with an extra Y chromosome. It’s been linked with mental retardation and violent tendencies. Because of its higher incidence in mental or penal institutions, some have linked it to criminal tendencies, though there may be a higher incidence in the general population that goes unreported.
Sexual maturation may be delayed, but normal. Increased height and decreased weight in adults. Lower cognitive abilities, delayed and poor language.
Prenatal hormones have organisational effects on the developing nervous system: structural and permanent changes, e.g., which influence the type of sexual behaviour that is expressed at a later stage. Whether a hormone can exert an activational effect depends on the way the brain’s been organised during development. E.g. female guinea pigs typically exhibit lordosis around ovulation.
This may occur by the gonadal steroids promoting dendritic growth on steroid sensitive cells, as has been shown to occur in vitro, hence competing for post-synaptic sites.
There's a sexing of parts of the brain involved in sexual behaviour, cognitive ability, and those that control our neuroendocrinology. There are critical periods for these organisational effects. If you treat pregnant ewes with testosterone treatment at different times, you can separately masculinise their hypothalamus, mounting behaviour, sexuality and urinary behaviour.
Cell size and density of dendrites differs in the preoptic area between males and females.
The medial preoptic area of the hypothalamus is important in male sexual behaviour. This area contains 5 times as many androgen receptors in males than females. Lesions eliminate copulatory behaviour in male rats, electrical or androgen stimulation facilitates sexual behaviour even after adult castration.
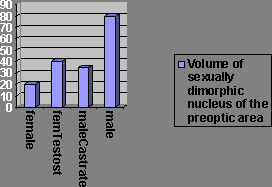
There’s a nucleus in the preoptic area, the sexually dimorphic nuclei, which is much larger in male than female rats. This too is an organisational effect from androgens being administered during critical periods (the 1st 10 days of life in rats).
Transplantation of the entire area from male newborn rats to female littermates enhances both homotypical and heterotypical adult sexual behaviours.
If females are treated with testosterone both prenatally and perinatally, their sexually dimorphic nucleus is as large as males’. DES, the synthetic oestrogen, has similar effects but the nuclei don’t grow quite as large, but nearly as large. Tamoxifen blocks the action of oestrogen. Cyproterone acetate blocks the action of testosterone. Males treated with cyproterone acetate show equally large sexually dimorphic nuclei, but with tamoxifen they have small nuclei, so oestradiol causes the masculinisation.
In humans, we have 4 dimorphic nuclei in the preoptic area, the interstitial nuclei of the anterior hypothalamus (INAH). INAH-3 and 4, especially INAH-3, are much larger in males, but not homosexuals.
Adult
activational effects are phasic, i.e. they depend upon the presence of
the hormone. Evidence for this includes the fact that
if you remove the testes from cockerels they fail to show masculine behaviour
such as crowing or fighting. If you transplant testes into the abdominal cavity
of castrated male cockerels, male behaviour returns. As the testes develop a
blood supply but no nerve supply, the signal must be transmitted through the
blood.
Increased levels of testosterone increase sexual motivation and behaviour in rats. If an adult male rat is castrated, i.e. a low level of testosterone, it will show no interest in a receptive female and will stop mating. If you inject testosterone in the adult-castrated rat normal sexual behaviour is restored. If capsules of androgens are placed in the anterior hypothalamus you get the best return.
Perinatally castrated rats will not show this activational effect. Re-administering androgens during this critical period will remasculinise them.
Androgenised brains show little behavioural response to oestrogens. Male rats castrated at birth can have lordosis activated hormonally. These rats will show reduced levels of male behaviour even if injected with testosterone. So the neural circuits controlling sexual behaviour are organised early in life.
Adult castration results in waning of first ability to ejaculate (impotence), then to get erect (within from weeks or years), and finally of sexual motivation. Impotence is not usually due to low testosterone levels (extra testosterone doesn’t help) but has a psychological cause.
The ventromedial hypothalamus is implicated in female sexual behaviour. The hypothalamic ventromedial nucleus contains a high number of oestrogen and progesterone receptors. Cell size differs in the ventromedial hypothalamus between males and females.
If lesioned, female rats don’t show lordosis even with oestrogen and progesterone injections simulating oestrus. Direct implants of oestrogen into this area encourages lordosis even after adult ovariectomy.
In females, oestrogen (i.e. an injection of oestradiol) upregulates the number of progesterone-receptors here. It doesn’t in males.
Oestrogen administration increases sexual receptivity in a dose dependent manner. You can activate lordosis by mimicking the hormonal effects at oestrus (around ovulation), i.e. give the female oestrogen for a few days, and then inject them with progesterone, even if they’re adult ovariectomised (though the hormones are metabolised and the behaviour’s short-lived) However, if they were administered testosterone just after birth (for rats) or are male you cannot activate lordosis with oestrogen and progesterone.
Humans are more complex; sexual activity is less dependent on levels of circulating hormones than most animals. Women are not just sexually active around oestrus. If you inject testosterone, both men and women increase sexual desire, but it doesn’t change sexual preference. Ovariectomy has little effect on women’s sexual behaviour, which is more affected by levels of androgens secreted by the adrenal glands (a tenth of that of men). Testosterone injections increase women’s sexual desires and removing the adrenal glands reduces it.
The preoptic area of the hypothalamus contains significantly more synapses in female rats than male. This effect is an organisational one under the control of the absence of testosterone perinatally.
Preoptic area neurons with oestrogen receptors project to the neurosecretory cells that produce gonadotropin-releasing hormone. This peptide controls the release of both luteinising hormone and follicle stimulating hormone from the anterior pituitary.
In females, ovarian follicle oestrogen activates the preoptic oestrogen sensitive cells, which causes a surge in the production of gonadotropin-releasing hormone.
In the androgenised brain, oestrogen and electrical stimulation of these preoptic cells does not result in luteinising hormone production from the pituitary.
The follicle-stimulating hormone causes the testes to produce sperm in males, and prepares the ovary for ovulation in females.
Luteinising
hormone makes the testes produce testosterone in males,
and triggers ovulation in females.
A hypothalamus exposed to androgens during the critical period, produces gonadotropin-releasing hormone, hence luteinising hormone, every 1-2 hours so testosterone is produced at a steady state. The female secretory pattern is over days. The circulating levels of gonadal steroids change across the menstrual/diurnal/annual cycle.
If
you replace ovaries in an ovariectomised rat it continues to ovulate, so
ovulation is under hormonal control.
If you implant ovaries in an adult male they won’t ovulate. If you castrate the rat at birth they will.
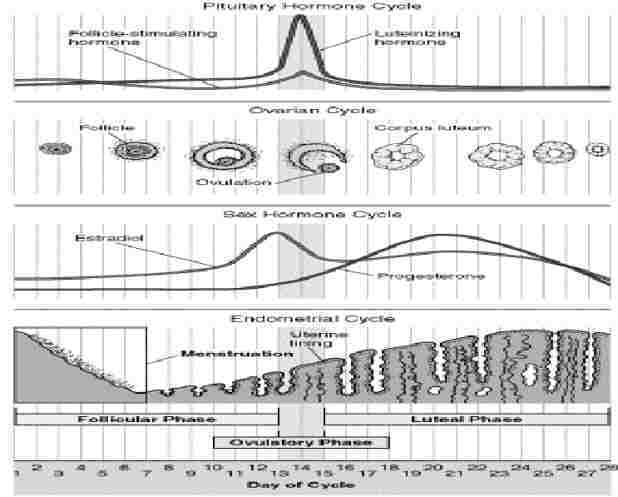
The menstrual cycle is related to the lining of the uterus, and differs from the oestrus cycle related to ovulation, though they show similarities. Both are under the control of pituitary gland hormones. The menstrual cycle is about 28 days.
There are 3 phases of the menstrual cycle: follicular, ovulatory and luteal. The combined pill has oestrogen to switch off the follicle-stimulating hormone, and progesterone to switch off luteinising hormone so the follicle can’t release the egg. (High enough levels would switch off the gonadotropin-releasing hormone.)
Follicular phase: The 1st day of the menstrual cycle is the 1st day of menstruation, and the start of the follicular phase, when the hypothalamus starts producing gonadotropin-releasing hormone, so the pituitary starts releasing follicle-stimulating hormone (which stimulates the development of up to 20 follicles in the ovary) and luteinising hormone. The follicle (sac protecting the ovum=unfertilised egg) releases oestrogen.
Ovulatory phase: About day 12, the level of oestrogen is high enough to switch of the pituitary’s production of follicle-stimulating hormone. The pituitary starts making a lot of luteinising hormone instead, which makes the follicle rupture and release the egg. The egg is viable for 12-24 hours. Sperm can stay alive for 24 hours (super-sperm up to 72). So there’s a 3-day possible pregnancy period.
Luteal phase: the follicle turns into the corpus luteum and produces progesterone (pro-gestation), which builds up the uterus lining and switches off the hypothalamus production of gonadotropin-releasing hormone, so levels of follicle-stimulating and luteinising hormone drop. If fertilisation happens, levels of progesterone stay high, else the corpus luteum shrivels and progesterone levels drop & the thick wall lining falls off.
Male brains are more likely to be lateralised. We have language and serial processing in the left hemisphere, and non-verbal and spatial processing in the right, e.g. face recognition, but the association between hemisphere and function is less strong in females according to neurological evidence. From 6 years boys prefer to use their left hand to discriminate shapes tactilely, girls not till 13. Language function transfers more readily to the right hemisphere after damage in females; their brains are more plastic. Developmental disorders associated with left hemisphere dysfunction, e.g. aphasia and autism, are more common in males.
Although it’s more or less the same size, because the female brain is smaller, the corpus callosum is relatively larger in women. Rats exposed to an enriched environment develop a thicker cerebral cortex. There’s a sex difference in that the right side is more likely to be thicker in male rats than female. In female rats you’ll find a number whose left cerebral cortex is larger than the right.
The bulbocavernosus nucleus motor neurons in the lower half of the spinal cord, which control the penis reflexes, has bigger and more neurons in the male than female rat. It atrophies in female development in the absence of androgens. In humans the bulbocavernosus motor neurons are found in Onuf’s nucleus in the spine, which is much bigger in males and its growth is sensitive to testosterone in the 26th week of gestation when male foetuses produce high levels of androgens. These neurons are partly under voluntary control and are used to urinate in men, and they innervate the muscles round the vagina opening in women.
Testosterone and oestrogen have differential effects on each hemisphere, e.g. suppressing one. There are activational effects of gonadal steroids on cognitive performance, e.g. spatial ability. In infant and adult male monkeys lesions of the orbital prefrontal cortex result in impaired spatial discrimination and delayed responses, but not in females under 15-18 months. Adult male monkeys have an advantage on spatial tasks.
There are steroid sensitive neurons in the frontal cortex of infant rats, whose number decreases by puberty.
Animals with seasonal aggression tend to fight when testosterone levels are highest. Adult castration reduces aggressive behaviour and injections restore it. Sensitivity to testosterone is determined organisationally in that perinatally castrated rats show little variation in aggression given testosterone.
Animals at the top of hierarchical societal structures tend to have highest testosterone levels, e.g. chickens and monkeys, yet the testosterone level of a monkey prior to joining a group doesn’t correlate with the level they’ll attain. Testosterone levels can increase as much as tenfold on attaining a high position. Success and victory increase testosterone levels while defeat and failure reduce them within about 24 hours in monkeys.
In humans, anticipating sex or watching an erotic film increases testosterone levels. Testosterone levels increase before a competition and remain high in the winners.
There’s a low correlation between testosterone levels and aggressiveness in humans, but e.g. the correlation is higher for low socio-economic status men.
The mature gonads of both sexes produce both androgens and oestrogens. Prepuberty is relatively anhormonal. Oestrogen and testosterone levels drop after birth and stay low throughout childhood.
Female monkeys exposed to androgens prenatally show more rough-and-tumble play, aggressiveness, less maternal imitative behaviour. Androgen-exposed prepubescents spend more time with boy monkeys and other androgen-exposed females.
The low level of oestrogen stops puberty happening in females.
The
hypothalamus controls the release of hormones from the gonads. Puberty is put
into motion by the release of gonadotropin-releasing hormone from the
hypothalamus, which stimulates the pituitary gland to produce luteinising
hormone and follicle-stimulating hormone.
At puberty male testes develop and produce testosterone, and causes the development of secondary characteristics: skeletal and muscle growth, body and pubic hair, voice deepening, ability to ejaculate.
In females sex hormones promote a fuller figure and wider hips.2 years after puberty girls start to menstruate, and it takes about another year before they release a mature ova.